SiDx's use of silicon photonics for blood testing
 Friday, July 30, 2021 at 10:07AM
Friday, July 30, 2021 at 10:07AM Part 4: Biosensor start-up, SiDx
A blood sample reveals much about a person’s health. But analysing the sample is complicated given its many constituents.
Identifying a user’s blood type is also non-trivial.
If a patient arriving at hospital needs a blood transfusion, the universal donor blood type, O negative, is administered. That’s because it takes too long - 45 minutes typically - to identify the patient’s blood type. This also explains the huge demand for O negative blood.
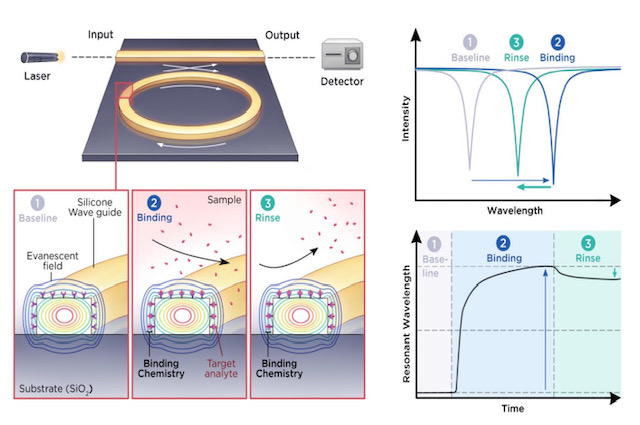 A laser lights the waveguide causing the ring to resonate. The blood sample then flows over the ring causing constituents to bind to the receptors. A rinse stage then removes specific bound components leaving the target constituent that has a signature wavelength shift. Source: SiDx.
A laser lights the waveguide causing the ring to resonate. The blood sample then flows over the ring causing constituents to bind to the receptors. A rinse stage then removes specific bound components leaving the target constituent that has a signature wavelength shift. Source: SiDx.
Identifying blood type promptly is what start-up SiDx set out to address with a platform based on a silicon photonics sensor. The resulting platform does more than just blood-type identification.
SiDx
The Seattle-based start-up was founded in 2017. By then, SiDx had a decade of research behind it, in silicon-photonics biosensors and the associated biochemistry.
SiDx had also started working with a blood centre in Seattle. Such centres source and sell blood to US hospitals.
“We were looking for an application that justified starting the company,” says Jonas Flueckiger, vice president of engineering at SiDx.
Flueckiger notes that silicon photonics is one of several ways to analyse biological materials. “It has advantages but there are alternatives,” he says. “You have to find an application where the advantages of silicon photonics can shine.”
Marketplace and challenges
Flueckiger splits the biosensor marketplace into three: centralised lab equipment, bedside and portable equipment, and home testing.
For centralised labs, what matters is the scale and the ability to perform parallel testing. Here, trained staff are required for sample preparation and operating the equipment.
The second category, bedside and portable systems, are compact and rugged platforms designed to deliver results quickly; SiDx’s testing system takes 12 minutes. Such platforms are newer and are the focus of SiDx and other silicon photonics biosensor start-ups.
“As for home tests, you don’t need a doctor’s office, you can do it yourself,” says Flueckiger.
 Jonas Flueckiger
Jonas Flueckiger
But medical diagnostics is a challenging market to enter. “The biggest challenge is that it is very, very hard to bring something new into the medical space,” says Flueckiger.
Hospitals are conservative establishments with rigid protocols that have test systems that doctors trust.
“Even though you show your system will be better, more efficient, faster, and the patient will be better served, it is still very hard to make a case to replace existing technology in a hospital,” says Flueckiger.
A new biosensor system must show it saves money, almost as important as demonstrating improved performance. “If your device is better but it costs more, that is not enough,” says Flueckiger.
Even if a start-up develops a system comparable in price, it must displace existing processes. And that raises a series of questions. Who does the testing? Where do the test results go? And who delivers the news to the patient?
“It is a complex picture and it is not just about technology,” says Flueckiger.
Ring resonators
SiDx’s silicon photonics platform measures refractive index changes in light caused by blood sample components attaching to ‘receptors’ placed on the sensor’s surface.
Biochemistry is required to design the receptors for blood analysis and is a core expertise of SiDx.
SiDx uses a laser coupled to a ring resonator. When blood sample constituents attach to the receptors on the ring resonator’s surface, the wavelength at which the sensor resonates changes. This shift in refractive index is used to identify the constituents. (See diagram above.)
A key benefit of the ring resonator approach is its tiny size. Multiple sensors can be integrated into a compact area allowing tests to be performed in parallel. Or as Flueckiger puts it, there is an ability to ask more than one question.
SiDx says it uses ring resonators but it is not detailing its design.
Most emerging integrated-photonics biosensing companies use a laser and ring resonator to read refractive index changes.
One way of get readings is using a tunable laser but alternative designs are possible such as using a fixed laser and tuning the resonator.
That is possible, says Flueckiger, but in a multiplexed design where multiple ring resonators are used, the electrical input-output for all the resonators gets tricky.
“Even for a single test, a single marker, you will have a negative control, a positive control, usually one or two more to make sure you have what you think you have,” says Flueckiger. “With bodily fluids like blood, it is complex and includes stuff that can interfere.”
Silicon photonics also enables label-free detection.
Here, only receptors are used to catch a blood constituent of interest. There is no need for labels with fluorescent attachments designed to link to the constituent.
But labelling improves the probability of identifying what is being looked for. Blood is so complex a sample that doctors may not want label-free testing for just this reason, the risk that another biomarker gives a similar response to what is being sought.
SiDx says sample preparation is key here. Rather than simply squirting the blood sample into the device, additional steps are used such as dilution or separating red blood cells from the serum with testing performed on either. Reagents can also be added to remove all the cells’ membranes.
Such sample preparation steps before label-free testing are important and non-trivial. “Photonics, that is the easy part,” says Flueckiger.
The resulting biosensor comprises optics and biology. Yet it requires a shelf life of 6-12 months. Another reason why medical biosensor design - and the biochemistry in particular - is challenging.
Blood testing and disease screening
Most people understand major blood types such as A, B, AB or O negative, says Flueckiger. But it is more complicated than that in that there are many subgroups. If they are assessed wrongly, it can prove harmful to a patient.
SiDx’s platform can perform blood typing and also Rhesus tests during pregnancy. Rhesus disease is caused by a certain mix of blood types between a pregnant mother and the unborn child.
SiDx sees blood typing as an entry to the market: to prove its technology before branching out. “Once you have blood typing and a sample, you can expand the test portfolio,” says Flueckiger.
The aim is to group tests in an offering that make sense. For example, testing for covid-19 but also testing for the common flu. Or, if a patient tests negative for covid-19, what else could it be? Testing that way and getting an answer avoids the need for a second test.
There are multiple ways to test for covid-19.
A PCR test looks for the DNA of the virus. Analysing a blood sample determines if a body’s immune response has developed antibodies to the virus. If so, it means the person has, or has had, covid-19. SiDx’s biosensor will also be able to test a person’s immune system after a vaccine and determine if a booster jab is needed.
SiDx’s system can detect DNA, but an issue is that DNA needs ‘amplification’; its levels are too small otherwise.
Using integrated photonics coupled with the right capture molecules on the surface allows what is captured to be detected. A DNA molecule is much smaller so other tricks are needed to measure it. As a result, antibodies are more commonly tested for because it is much easier, says Flueckiger.
Prospects
SiDx along with other silicon photonics biosensor companies such as Genalyte, SiPhox, Rockley Photonics and Antelope DX, all received recent funding rounds. SiDx has raised a total of $3 million in funding and $2 million in research funding.
Is this not a vote of confidence in what is a tough market to crack?
There is venture money but it is hard to come by, says Flueckiger. Developing a medical device takes time, a minimum of five years before getting somewhere. If a company starts from scratch, the development time is longer than what venture capitalists are happy with.
Companies pursuing blood testing also can expect greater scrutiny given the story of private company, Theranos, whose claims about developing a breakthrough blood analysis system proved false.
Venture capitalists recognise the potential of benchtop devices but their concern is making money and how quickly a start-up can multiply their investment.
“Unless you can show hockey-stick growth, it’s a tough sell,” says Flueckiger. “These are long-term investments, not like a software company.”
That said, the covid-19 pandemic has helped. People now understand the important role such diagnostic equipment can play. They recognise how long it takes and that if money is thrown at the problem, device development can be accelerated.
Despite the challenges, Flueckiger is upbeat. “We have made lots of progress,” he says. “We have proven to ourselves that our technology works.” SiDx says there are developments that bring its platform closer to market that it cannot disclose at present.
The coronavirus pandemic also provided the company with a motivational boost to launch a product that is far easier to use.
SiDx did consider shifting its focus to address covid-19 testing but the pandemic occurred a year too early. “If it happened now, we would be in a lot better position to turn around very quickly with limited money and have a test ready,” says Flueckiger.
SiDx says that its conversations with investors generate excitement but they want proof of a return.
“You go into this knowing you have a long runway - the next five years will be hard - and then there is the question of whether you will be successful or not,” says Flueckiger.



Reader Comments